by Ella Woodruff
Friday Harbor Laboratories
When I think back to the beginning of the summer of 2021, I couldn’t have imagined how my research experience at Friday Harbor labs (FHL) would turn out. I am an undergraduate student at Carleton College in Minnesota and I came out to Friday Harbor last summer to gain experience in marine research, where I was introduced to Drs. Adam Summers and Karly Cohen. My original project was going to focus on studying the overlapping and heavy armor of poacher fishes. However, Karly – who is currently studying color in Pacific Spiny Lumpsuckers (Eumicrotremus orbis) – had a large dataset to work with, and was glad to have my help on the topic of armor.
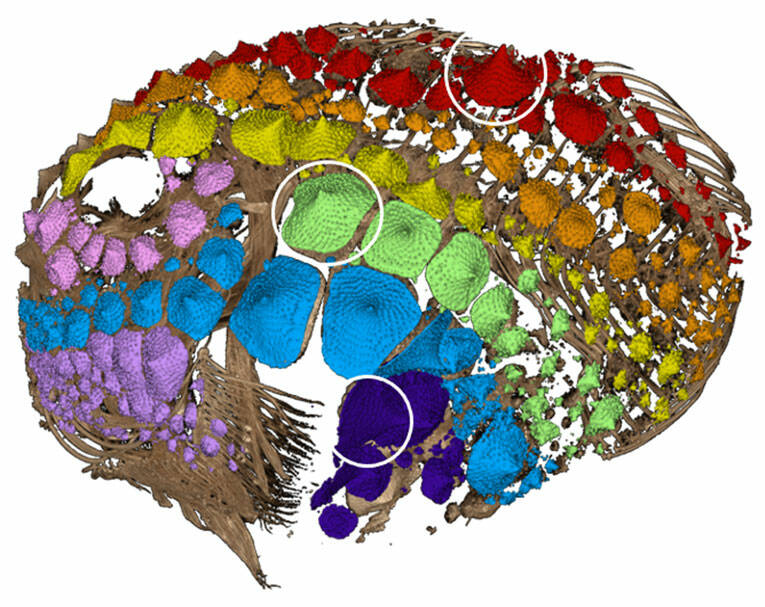
Armor has evolved in fishes many different times. In addition to the lightweight and flexible scale armor familiar to anyone who has prepared a fish for dinner, there are heavy plates, bony knobs, and mineralized spikes in some species. Heavily armored fishes have to balance protectiveness vs. mobility. For example, Pacific Spiny Lumpsuckers are small, rotund, heavily-armored fishes that live in the intertidal and subtidal waters of the north Pacific ocean and are a common find off the docks at FHL (Figure 1). How can little, round fishes move when they have a heavy coating of armor? Our goal was to understand the growth and mineral investment in lumpsucker armor. Earlier in the year, Karly and Jonathan Huie had CT (computerized tomography) scanned over 40 preserved individual fish on loan from the University of Washington’s Burke Museum, ranging in size from 9-90 mm. This gave me a large data set to see what the armor is made of, how it grows as the fish grows, and whether and how it gets damaged.
After measuring and studying more than 5000 pieces of armor (bigger lumpsuckers can have up 400 pieces of armor whereas smaller fish may only have 50), we determined it is composed of individual cone-shaped pieces that cover most of their body (Figure 2). Throughout this project, we struggled with what to call these cones. More than scales and distinct from bony plates, we settled on “odontodes,” reflecting the toothy material (enamel) and development of the armor. The most famous odontodes are those that cover sharks. These dermal denticles are tooth-like structures, and regardless of where they are found, any dentine+enamel protuberance is an odontode as well. By examining the lumpsucker odontodes in detail using SEM (scanning electron microscopy) and CT, we found that their armor can be damaged by both abrasion and impact (Figure 3). Scraping the surface (abrasion) and bouncing into rocks (impact) are regular parts of the life of the lumpsucker – in the end we discovered that these little fish are covered in teeth that help protect them in the turbulent subtidal!
When I began measuring the material investment of lumpsucker armor, I expected there to be similar results to what had been recorded in poachers; though this may have been due to my own ignorance on the subject of fish armor. Investment looks at how much mineral is in the skeleton of a fish vs. the armor. Sometimes armor is super heavy – imagine the garb of the Holy Roman Emperor Ferdinand. Other times, armor is light; and while not great for defense can serve other purposes such as camouflage or display. Lumpsucker armor is not heavy but it is protective. While only big lumpsuckers invest more material in their armor than in their skeleton, the enamel-based armor is a lightweight solution to the problem of abrasion. Enamel is great at resisting abrasion but is not nearly as heavy as the bony plates that cover other fishes, for example the Northern Spearnose Poacher pictured in the study linked early in this paragraph. Lumpsuckers are poor swimmers at best, so this lightweight solution may be a compromise to prevent further restriction of their mobility.
This project immersed me in new methods and techniques. On my first day I learned how to use the micro-CT scanner, and my first week was spent helping with another project to scan deep-sea fishes. Though I did not scan any of the Pacific Spiny Lumpsuckers used in this project myself, knowing how the data were collected gave me a more comprehensive view of the scientific process and helped me with the data manipulation. My introduction to the lumpsucker project started with familiarizing myself with specialized, free, open-source software called 3DSlicer and the SlicerMorph extension, segmentation, and quantification. I spent weeks on the computer using 3DSlicer to analyze these fish before I was even able to see them in the flesh. Eventually we got a new shipment of lumpsuckers from the Burke Museum to enable us to do scanning electron microscopy of the armor. Having looked at scans of the fish on the computer for weeks, seeing them in person for the first time was shocking, and I came to love these cute fish even more after holding them in my hand.
Beyond the science, I was exposed to other new experiences this summer such as working with others in a data-intensive environment and living at a marine lab like FHL. This project and my time at the Labs would not have been possible without access to the Karel F. Liem Bioimaging facility, the Eugster Endowed Student Research Fund, and the Carleton College Career Center. I also benefited from interactions with the 2021 FHL REU cohort, who supported me through my work, though I was not an official member of the group.
If you want to know more about our study, you can find it recently published (and on the cover!) of the Journal of Morphology and recently covered by the New York Times and the University of Washington press!